Abstract
Introduction
Positron emission tomography (PET) positivity before autologous stem cell transplantation (ASCT) predicts poor prognosis in relapsed/refractory (rel/ref) classical Hodgkin lymphoma (cHL). However, there is limited data regarding the value of pre-ASCT residual metabolic tumor volume (rMTV) assessed by PET to predict post-ASCT outcomes. We aimed to evaluate the role of pre-ASCT Deauville score (DS) combined with rMTV in patients with rel/ref cHL who underwent salvage ASCT, and also to establish a risk model integrating pre-ASCT DS, rMTV, and other clinical risk factors.
Methods
This is a retrospective cohort study using clinical data of patients with rel/ref cHL identified from the lymphoma database and the ASCT database of the Princess Margaret Cancer Center, Toronto, Canada. Following criteria were required: (1) patients (≥18 yrs) with histologically confirmed rel/ref cHL between January 2014 to March 2019; (2) treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) or ABVD-equivalent regimens; (3) chemosensitive disease after salvage therapy; (4) restaging PET before ASCT clearance. Pre-ASCT PET scans were assessed using the Deauville 5-point scale, and rMTV was computed in the scans with DS 4 or 5 using the 41% maximum standardized uptake value threshold method. Receiver operating characteristics analysis was used to determine an optimal cutoff of rMTV 41% for event prediction. The primary endpoint was event-free survival (EFS), defined as time form ASCT to first documented disease progression, any new lymphoma therapy, death from any cause, or last follow-up.
Results
A total of 106 patients fulfilled the eligibility criteria and were included. Median age was 33.5 yrs (range, 18-70), and 72 (68%) were male. The cohort characteristics at time of progression were 55 (52%) patients with advanced stage, 28 (26%) with B symptoms, 39 (37%) with extranodal disease, and 39 (37%) with primary refractory disease, defined as achieving less than a complete response, disease progression during first-line therapy, or time to relapse (TTR) 3 months or less. All patients received gemcitabine, dexamethasone, cisplatin (GDP) regimen as salvage therapy. Based on the response to GDP therapy, 24 patients required second (N=20) or third (N=4) salvage regimens before ASCT (brentuximab vedotin [BV] alone or combination with bendamustine [N=16], pembrolizumab [N=9], and mini-BEAM [N=3]). 32 patients (30%) received planned pre- or post-ASCT involved field radiation for DS 4-5 lesions. 4 patients received post-ASCT BV consolidation.
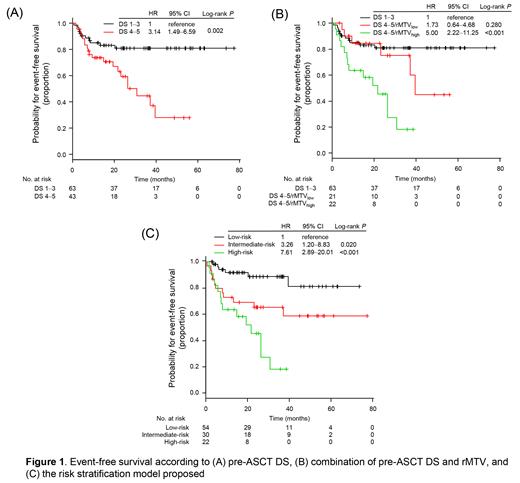
With a median follow-up of 26.2 months (interquartile range [IQR], 14.4-49.2), 2-year EFS and overall survival rates were 72.1% and 93.1%, respectively. Pre-ASCT DS was determined as 1-3 (N=63, 59%) and 4-5 (N=43, 41%). None of the patients with DS 5 had new lesions. Patients with pre-ASCT DS 1-3 had significantly better EFS than those with DS 4-5 (2-year; 80.8% vs 59.2%; P=0.002; Fig 1A).
Median rMTV 41% of the 43 patients with DS 4-5 was 5.1 cm 3 (IQR, 2.0-24.7). The optimal cutoff of rMTV 41% was 4.4 cm 3. Patients with rMTV low (< 4.4cm 3, N=21) had better EFS than those with rMTV high (N=22; 2-year, 75.0% vs 45.4%; P=0.009), but had similar 2-year EFS when compared with patients with DS 1-3 (80.8%, P=0.280; Fig 1B)
In the multivariable analysis for EFS, a combined assessment of pre-ASCT DS/rMTV 41% and rel/ref status at time of progression were independently associated with EFS (for DS 4-5/rMTV low, hazard ratio [HR] 1.58; for DS 4-5/rMTV high, HR 4.72; P<0.001: for primary refractory disease, HR 2.26; P=0.026). Based on these results, we stratified patients into 3 groups according to the post-ASCT outcomes: low-risk (relapsed disease [i.e., TTR > 3 months] and either pre-ASCT DS 1-3 or DS 4-5/rMTV low, N=54), intermediate-risk (refractory disease and either pre-ASCT DS 1-3 or DS 4-5/rMTV low, N=30), and high-risk (pre-ASCT DS 4-5/rMTV high irrespective of rel/ref status, N=22) groups. The risk model was significantly associated with EFS (intermediate vs low, HR 3.26, 95%CI 1.20-8.83, P=0.020; high vs low, HR 7.61, 95% CI 2.89-20.01, P<0.001; Fig 1C).
Conclusion
We propose a risk stratification model integrating a combination of DS and rMTV 41% on pre-ASCT PET and rel/ref status at time of progression, which allow discrimination of post-ASCT outcomes in patients with rel/ref cHL. The model may serve as a tool to facilitate risk-stratified treatment decisions.
Metser: POINT Biopharm Inc: Consultancy. Prica: Astra-Zeneca: Honoraria; Kite Gilead: Honoraria. Crump: Roche: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding; Kyte/Gilead: Membership on an entity's Board of Directors or advisory committees. Kuruvilla: Seattle Genetics: Honoraria; Amgen: Honoraria; Antengene: Honoraria; AbbVie: Honoraria; Janssen: Honoraria, Research Funding; BMS: Honoraria; Medison Ventures: Honoraria; TG Therapeutics: Honoraria; AstraZeneca: Honoraria, Research Funding; Gilead: Honoraria; Incyte: Honoraria; Karyopharm: Honoraria, Other: Data and Safety Monitoring Board; Roche: Honoraria, Research Funding; Novartis: Honoraria; Merck: Honoraria; Pfizer: Honoraria. Kridel: Gilead Sciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal